- Home
- About Us
- Professionals
- Professionals
- Professionals
- Services
- Services
- Practice Areas
- Information Security
- News
- Database
- Database
- References
- Foundin Cases
- Related Websites
- Contact
Revised Chinese Patent Examination Guidelines Took Effect from 15 January, 2021 (PART II)
Written by Xiao
Han
The
China National Intellectual Property Administration (“CNIPA”) recently
announced the "Revised Patent Examination Guidelines", which came
into force on January 15, 2021. This is a major development in the field of IP
industry in China. Although the “Revised Guideline" is not a formal source
of law, it can be used as a reference in courts’ decisions provided that there
are no conflicts with the "Chinese Patent Law" and its implementation
rules. Therefore, its amendment is of great significance to the administrative
and judicial procedures on patent-related matters.
In
this article, we just focus on the amendments regarding novelty of compounds,
inventive steps of compounds, deposition, monoclonal antibodies, and inventive
steps in biotechnology.
Novelty of
Compounds
The
“Revised Guidelines" states: “where a patent application claims a
compound, if the chemical name,
molecular formula (or structural formula) and other structural information of
the compound is recorded in a reference so that those skilled in the art
believe that the claimed compound has been disclosed, the compound is not
novel, unless the applicant can provide evidence to prove that the compound
cannot be obtained before the filing date.”
In other words, even if the reference has disclosed the same physical
and chemical parameters as the claimed compound, it cannot be directly inferred
that the compound is not novel.
In
addition, according to the "Revised Guidelines", when the structural
similarities and differences between the claimed compounds and reference cannot
be determined, physical and chemical
parameters, preparation and experimental effects, and other factors should
be combined and considered to determine whether the compounds can be predicted
as substantially the same by the skilled in the art. On the contrary, the
applicant can prove the novelty of the compound by proving the structural
difference between the claimed compound and the reference.
Inventive Steps of
Compounds
The
"Revised Guidelines" has made major changes to the examination of
inventive steps of compounds, that is, regardless of whether the compound is
similar in structure to the existing compound, a unified "problem-solution-approach"
is used to determine whether a compound is inventive.
The
"Revised Guidelines" provides the following examples under this
topic:
[Example
1]
Prior
art:
(Ia)
Application:
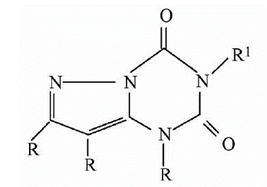
(Ib)
(Ib)
and (Ia) have different core structures, but they have the same use. Those
skilled in the art generally believe that compounds with similar structures
have the same or similar uses, and similar structures generally mean that the compounds
have the same basic core part or basic ring. In the prior art, there is no
technical inspiration about modifying the basic ring of (Ia) to obtain (Ib)
while maintaining the same use, therefore, (Ib) meets the requirement of
inventive steps.
[Example
2]
Prior
art: H2N-C6H4-SO2NHR1
(IIa)
Application:
H2N-C6H4-SO2-NHCONHR1
(IIb)
(IIb)
is obtained by inserting -CONH- into the structural fragment NHR1 of
(IIa). (IIa) and (IIb) have different uses as (IIa) is a sulfa antibiotic while
(IIb) is sulfonyl acyl urea and an anti-diabetic drug. Those skilled in the art
have no motivation to modify R1 in the sulfa antibiotic into CONHR1 to
obtain the anti-diabetic drugs. Therefore, (Ib) meets the requirement of
inventive steps.
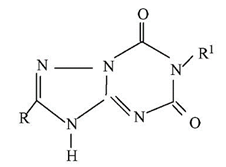
Deposition
Guangdong
Culture Collection Center (GDMCC) located in Guangzhou is added.
Monoclonal
Antibodies
Regarding
the drafting of monoclonal antibody claims, the "Revised Guidelines"
adds a limitation method of “limiting by structural features”.
It
is stipulated in the current Guidelines that monoclonal antibody claims can be
defined by the hybridoma from which it is produced. However, with the maturity
and popularization of monoclonal antibody sequencing technology, it has become
easier to obtain structural information of monoclonal antibodies and it is
possible to define monoclonal antibodies by structural features.
The
"Revised Guidelines" provides the following examples under this
topic:
[Example]
1. A
monoclonal antibody of antigen A, comprising VHCDR1, VHCDR2, and VHCDR3 shown
in amino acid sequences of SEQ ID NOs. 1-3, and VLCDR1 VHCDR2, and VHCDR3 shown
in amino acid sequences of SEQ ID NOs. 4-6.
2. A monoclonal
antibody of antigen A produced by hybridoma of CGMCC Deposition No: XXXX.
Inventive Steps in
Biotechnology
Regarding
the inventive steps of biotechnology, the "Revised Guidelines"
discarded the over-absolute requirement of "unexpected technical effect"
and returned to the "problem-solution-approach" for examination. In
addition, the "Revised Guideline" also enriched subject matters and
added new forms of biotechnology such as "peptides or proteins".

 adm
adm

